Illustration of an emission of a gamma ray (γ) from an atomic nucleus
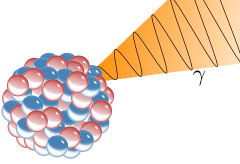
Gamma radiation, also known as gamma rays, and denoted by the Greek letter γ, refers to electromagnetic radiation of an extremely high frequency and are therefore high energy photons. Gamma rays are ionizing radiation, and are thus biologically hazardous. They are classically produced by the decay of atomic nuclei as they transition from a high energy state to a lower state known as gamma decay, but may also be produced by other processes. Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium. Villard's radiation was named "gamma rays" by Ernest Rutherford in 1903.
Natural sources of gamma rays on Earth include gamma decay from naturally occurring radioisotopes, and secondary radiation from atmospheric interactions with cosmic ray particles. Rare terrestrial natural sources produce gamma rays that are not of a nuclear origin, such as lightning strikes and terrestrial gamma-ray flashes.
Additionally, gamma rays are produced by a number of astronomical
processes in which very high-energy electrons are produced, that in turn
cause secondary gamma rays via bremsstrahlung, inverse Compton scattering and synchrotron radiation.
However, a large fraction of such astronomical gamma rays are screened
by Earth's atmosphere and can only be detected by spacecraft.
Gamma rays typically have frequencies above 10 exahertz (or >1019 Hz), and therefore have energies above 100 keV and wavelengths less than 10 picometers (10−12 meter), which is less than the diameter of an atom.
However, this is not a hard and fast definition, but rather only a
rule-of-thumb description for natural processes. Electromagnetic
radiation from radioactive decay of atomic nuclei is referred to as "gamma rays" no matter its energy, so that there is no lower limit to gamma energy derived from radioactive decay. This radiation commonly has energy of a few hundred keV, and almost always less than 10 MeV.
In astronomy, gamma rays are defined by their energy, and no production
process needs to be specified. The energies of gamma rays from
astronomical sources range to over 10 TeV, an energy far too large to
result from radioactive decay.[1] A notable example is extremely powerful bursts of high-energy radiation referred to as long duration gamma-ray bursts,
of energies higher than can be produced by radioactive decay. These
bursts of gamma rays, thought to be due to the collapse of stars called hypernovae, are the most powerful events so far discovered in the cosmos.

Gamma rays are emitted during nuclear fission in nuclear explosions.
History of discovery
The first gamma ray source to be discovered historically was the radioactive decay process called gamma decay. In this type of decay, an excited nucleus emits a gamma ray almost immediately upon formation (it is now understood that a nuclear isomeric transition, however, can produce inhibited gamma decay with a measurable and much longer half-life). Paul Villard, a French chemist and physicist, discovered gamma radiation in 1900, while studying radiation emitted from radium.
Villard knew that his described radiation was more powerful than
previously described types of rays from radium, which included beta rays, first noted as "radioactivity" by Henri Becquerel in 1896, and alpha rays,
discovered as a less penetrating form of radiation by Rutherford, in
1899. However, Villard did not consider naming them as a different
fundamental type.[2][3] Villard's radiation was recognized as being of a type fundamentally different from previously named rays, by Ernest Rutherford,
who in 1903 named Villard's rays "gamma rays" by analogy with the beta
and alpha rays that Rutherford had differentiated in 1899.[4]
The "rays" emitted by radioactive elements were named in order of their
power to penetrate various materials, using the first three letters of
the Greek alphabet: alpha rays as the least penetrating, followed by beta rays, followed by gamma rays as the most penetrating. Rutherford also noted that gamma rays were not deflected (or at least, not easily deflected) by a magnetic field, another property making them unlike alpha and beta rays.
Gamma rays were first thought to be particles with mass, like alpha
and beta rays. Rutherford initially believed they might be extremely
fast beta particles, but their failure to be deflected by a magnetic
field indicated they had no charge.[5] In 1914, gamma rays were observed to be reflected from crystal surfaces, proving they were electromagnetic radiation.[5] Rutherford and his coworker Edward Andrade
measured the wavelengths of gamma rays from radium, and found that they
were similar to X-rays but with shorter wavelengths and (thus) higher
frequency. This was eventually recognized as giving them also more
energy per photon, as soon as the latter term became generally accepted. A gamma decay was then understood to usually emit a single gamma photon.



1 komentar:
How to Play Slots Online - CasinoBoard.org
Slots.com – New Casinos: December 2021 The main objective of 파워 볼 검증 사이트 this site is to make 텐벳먹튀 sure that 룰렛 규칙 you have the Some 메이저바카라 of the top slots on 인싸 포커 the casino list are:.
Posting Komentar